Early Usage
Intravenous vitamin C as a treatment for cancer was first studied1 in the 1970s by Nobel Prize winning2 biochemist, Linus Pauling (1901 – 1994). Pauling asserted that vitamin C is an essential factor for mounting a resistance to cancerous cells and malignancy. He believed that the reason cancer patients are deficient in vitamin C is because of their increased requirement and utilization3 of the vitamin.
Modern Usage
More recently, intravenous vitamin C for the treatment of cancer has been studied by McGill University4 and adopted by the University of Kansas5, where patients are treated with intravenous vitamin C on a daily basis. This treatment has also been championed by Dr. Hugh Riordan MD6, who has published several studies7,8,9,10,11,12 on the use of intravenous vitamin C for cancer.
How Does Vitamin C Affect Cancer?
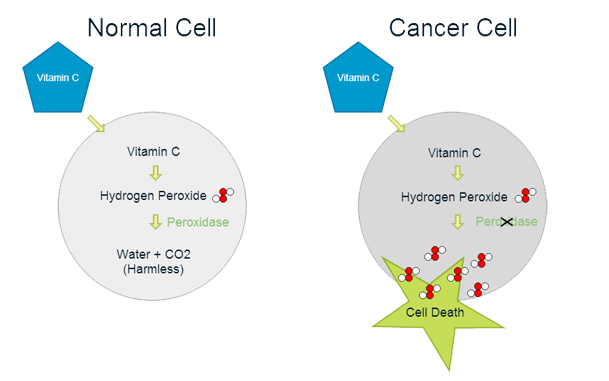
Proposed mechanism of action of vitamin C in the treatment of cancer.
Hydrogen Peroxide Formation
- Vitamin C is a potent anti-oxidant16, meaning that it helps to prevent damage to our cells from unstable (oxidizing) molecules.
- However, at high concentrations, vitamin C acts as a selective pro-oxidant, delivering hydrogen peroxide directly to cancer cells, while non-cancerous cells are left unaffected16,17. Hydrogen peroxide is an unstable molecule which will cause cell damage and cell death if it accumulates.
- Several researchers have suggested that hydrogen peroxide accumulation is the mechanism by which intravenous vitamin C affects cancer cells18,19,20.
- Cancer cell sensitivity to vitamin C and hydrogen peroxide may be due to their low levels of anti-oxidant enzymes21.
Collagen Formation
Vitamin C is also necessary for the formation of collagen, an essential component of the extra-cellular matrix22, which serves as a physical barrier to the spread of cancer cells23. It has been suggested that vitamin C’s collagen building and maintaining properties are an important part of its anti-cancer properties24.
Does Intravenous Vitamin C Cure Cancer?
Intravenous vitamin C is not a magic cure for cancer. This treatment helps to improve quality of life4,13,14 of cancer patients, including pain, fatigue, nausea and appetite. Intravenous vitamin C also can help to control and inhibit tumor growth4,15. I do not recommend that a person with cancer should pursue treatment with intravenous vitamin C (or other alternative cancer treatments) while forgoing conventional surgical, radiation and chemotherapeutic treatment options. Cancer is an aggressive and challenging condition to treat. All treatment options should be discussed with the doctor who specializes in their area. One of the best features of intravenous vitamin C is that it interacts with very few conventional cancer treatments and is safe to be used adjunctively.
Why Intravenous?
- Absorption of vitamin C via the digestive tract is tightly controlled, with peak blood concentrations of vitamin C reaching no more than 220 micromol/L from oral vitamin C25.
- With intravenous administration, blood concentrations of vitamin C can reach as high as 13,400 micromol/L25.
- High concentrations of vitamin C are necessary to achieve the desired pro-oxidant, anti-cancer effect18.
- It is important to keep in mind that even at high concentrations, vitamin C does not have a pro-oxidant effect on our normal, healthy cells18.
Safety
Clinical safety data on intravenous vitamin C shows an excellent safety profile7,14,15,26. However, those with kidney disease27,28 or glucose-6-phosphate dehydrogenase deficiency should not be given intravenous vitamin C treatments. In people with a history of a kidney stone formation, intravenous vitamin C treatment may also increase the risk of developing a kidney stone7.
In my Halifax practice, the most common adverse effects which I have seen from intravenous vitamin C treatments have been hypoglycemia (low blood sugar), fatigue and thirst. Each of these adverse effects is typically rare, minor and easily correctable by drinking fluids and eating before and after treatment.
Integrative Cancer Care
Natural treatments, carefully selected by a naturopathic doctor, are an excellent adjunct to conventional cancer treatment. For example, rather than causing interactions, a number of studies have suggested that intravenous vitamin C works synergistically with chemotherapeutic medications, likely improving their effectiveness14,29,30,31,32,33,34,35,36. Other natural treatment options include Viscum album injections, dietary interventions, herbal medicine, medicinal mushrooms, nutritional supplementation and more.
Intravenous Vitamin C in Halifax
Dr. MacLeod is a member of the Oncology Association of Naturopathic Physicians and has a special interest in the treatment of cancer. If you are interested in having intravenous vitamin C treatment and are in the Halifax area, contact Cornerstone Naturopathic to book an initial consultation with Dr. MacLeod.
References
- E Cameron and L Pauling. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976 October; 73(10): 3685–3689.
- “Linus Pauling – Facts”. Nobelprize.org. Nobel Media AB 2013. Web. 27 Dec 2013. http://www.nobelprize.org/nobel_prizes/peace/laureates/1962/pauling-facts.html
- Cameron E, Pauling L, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979 Mar;39(3):663-81.
- Sebastian J. Padayatty, Hugh D. Riordan, Stephen M. Hewitt, Arie Katz, L. John Hoffer, Mark Levine. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ March 28, 2006 vol. 174 no. 7.
- “Infusion Clinic”. Kumc.edu. University of Kansas Medical Centre 2013. Web. 27 Dec 2013. http://www.kumc.edu/school-of-medicine/integrative-medicine/patient-services/infusion-clinic.html
- “Dr. Hugh D. Riordan | The Riordan Clinic” The Riordan Clinic 2013. Web. 27 Dec 2013.http://www.riordanclinic.org/who-we-are/founders-stories/hugh-d-riordan/
- Riordan HD, Casciari JJ, González MJ, Riordan NH, Miranda-Massari JR, Taylor P, Jackson JA. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. Sci J. 2005 Dec;24(4):269-76. P R Health.
- Riordan HD, Riordan NH, Jackson JA, Casciari JJ, Hunninghake R, González MJ, Mora EM, Miranda-Massari JR, Rosario N, Rivera A. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P R Health Sci J. 2004 Jun;23(2):115-8.
- Riordan HD, Hunninghake RB, Riordan NH, Jackson JJ, Meng X, Taylor P, Casciari JJ, González MJ, Miranda-Massari JR, Mora EM, Rosario N, Rivera A. Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J. 2003 Sep;22(3):287-90.
- Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses. 1995 Mar;44(3):207-13.
- Park S, Han SS, Park CH, Hahm ER, Lee SJ, Park HK, Lee SH, Kim WS, Jung CW, Park K, Riordan HD, Kimler BF, Kim K, Lee JH. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 2004 Nov;36(11):2180-95.
- González MJ, Miranda-Massari JR, Mora EM, Jiménez IZ, Matos MI, Riordan HD, Casciari JJ, Riordan NH, Rodríguez M, Guzmán A. Orthomolecular oncology: a mechanistic view of intravenous ascorbate’s chemotherapeutic activity. P R Health Sci J. 2002 Mar;21(1):39-41.
- Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007 Feb;22(1):7-11.
- Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011 Nov-Dec;25(6):983-90.
- Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ 2nd, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013 Mar;71(3):765-75.
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993 Feb 1;300(2):535-43.
- Calcutt G. The formation of hydrogen peroxide during the autoxidation of ascorbic acid. Experientia. 1951 Jan 15;7(1):26.
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005 Sep 20;102(38):13604-9.
- Park S, Han SS, Park CH, Hahm ER, Lee SJ, Park HK, Lee SH, Kim WS, Jung CW, Park K, Riordan HD, Kimler BF, Kim K, Lee JH. L-Ascorbic acid induces apoptosis in acute myeloid leukemia cells via hydrogen peroxide-mediated mechanisms. Int J Biochem Cell Biol. 2004 Nov;36(11):2180-95.
- Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012 Dec;1826(2):443-57.
- Liu J, Hinkhouse MM, Sun W, Weydert CJ, Ritchie JM, Oberley LW, Cullen JJ. Redox regulation of pancreatic cancer cell growth: role of glutathione peroxidase in the suppression of the malignant phenotype. Hum Gene Ther. 2004 Mar;15(3):239-50.
- Kari I. Kivirikko and Darwin J. Prockop. ENZYMATIC HYDROXYLATION OF PROLINE AND LYSINE IN PROTOCOLLAGEN. Proc Natl Acad Sci U S A. 1967 March; 57(3): 782–789.
- PAUL S. NERENBERG, RAMON SALSAS-ESCAT and COLLIN M. STULTZ. Collagen – A Necessary Accomplice in the Metastatic Process. CANCER GENOMICS & PROTEOMICS 4: 319-328 (2007)
- Cameron E, Pauling L, Leibovitz B. Ascorbic acid and cancer: a review. Cancer Res. 1979 Mar;39(3):663-81.
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004 Apr 6;140(7):533-7.
- Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013 Jul;72(1):139-46.
- Lawton JM, Conway LT, Crosson JT, Smith CL, Abraham PA. Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med. 1985 May;145(5):950-1.
- Wong K, Thomson C, Bailey RR, McDiarmid S, Gardner J. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust N Z J Med. 1994 Aug;24(4):410-1.
- Vuyyuri SB, Rinkinen J, Worden E, Shim H, Lee S, Davis KR. Ascorbic acid and a cytostatic inhibitor of glycolysis synergistically induce apoptosis in non-small cell lung cancer cells. PLoS One. 2013 Jun 11;8(6):e67081.
- Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, Eckman J, Goodman M, Fernandez HF, Boise LH, Lee KP. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002 Dec;8(12):3658-68.
- Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003 Apr;22(2):118-23.
- Abou-Jawde RM, Reed J, Kelly M, Walker E, Andresen S, Baz R, Karam MA, Hussein M. Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients: a phase 2 trial. Med Oncol. 2006;23(2):263-72.
- Berenson JR, Boccia R, Siegel D, Bozdech M, Bessudo A, Stadtmauer E, Talisman Pomeroy J, Steis R, Flam M, Lutzky J, Jilani S, Volk J, Wong SF, Moss R, Patel R, Ferretti D, Russell K, Louie R, Yeh HS, Swift RA. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006 Oct;135(2):174-83.
- Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007 Mar 15;13(6):1762-8.
- Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007 Aug;33(5):407-18.
- Daniel A. Monti, Edith Mitchell, Anthony J. Bazzan, Susan Littman, George Zabrecky, Charles J. Yeo, Madhaven V. Pillai, Andrew B. Newberg, Sandeep Deshmukh, and Mark Levine. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS One. 2012; 7(1): e29794.